Count Alessandro Volta | |
|---|---|
 | |
| Born | (1745-02-18)18 February 1745 Como, Duchy of Milan |
| Died | 5 March 1827(1827-03-05) (aged 82) Como, Lombardy-Venetia, Austrian Empire |
| Nationality | Italian |
| Known for |
|
| Spouse | Teresa Peregrini |
| Children | 3 Sons |
| Awards |
|
| Scientific career | |
| Fields | Physics and chemistry |
| Articles about |
| Electromagnetism |
|---|
 |
|
|
Electrostatics
|
|
Magnetostatics
|
|
Electrodynamics
|
|
Electrical network
|
|
Magnetic circuit
|
|
Covariant formulation
|
|
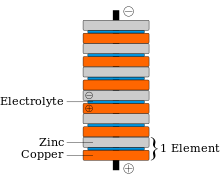
Alessandro Giuseppe Antonio Anastasio Volta (/ˈvoʊltə, ˈvɒltə/, Italian: [alesˈsandro ˈvɔlta]; 18 February 1745 – 5 March 1827) was an Italian physicist and chemist who was a pioneer of electricity and power and is credited as the inventor of the electric battery and the discoverer of methane. He invented the voltaic pile in 1799, and reported the results of his experiments in 1800 in a two-part letter to the president of the Royal Society. With this invention Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of electrochemistry.
Volta also drew admiration from Napoleon Bonaparte for his invention, and was invited to the Institute of France to demonstrate his invention to the members of the institute. Throughout his life, Volta enjoyed a certain amount of closeness with the emperor who conferred upon him numerous honours.
Volta held the chair of experimental physics at the University of Pavia for nearly 40 years and was widely idolised by his students. Despite his professional success, Volta was inclined towards domestic life and this was more apparent in his later years when he tended to live secluded from public life and more for the sake of his family. He died in 1827 from a series of illnesses which began in 1823. The SI unit of electric potential is named the volt in his honour.